 ... Are getting better and better.
... Are getting better and better.Researchers reporting in the July issue of Diabetes Care found that for their Type 1 patients, inhaled insulin was comparable to injected insulin for meal-time doses. Subjects still injected a basal dose twice a day, but used an inhaler when bolus insulin was needed.
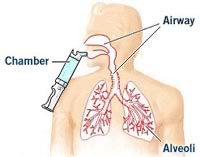
 The inhaled insulin used in the study was ExuberaTM, a short-acting, dry powder, human insulin developed in a joint venture by Sanofi-Aventis and Pfizer. The inhalation device (shown) was developed by Nektar Therapeutics.
The inhaled insulin used in the study was ExuberaTM, a short-acting, dry powder, human insulin developed in a joint venture by Sanofi-Aventis and Pfizer. The inhalation device (shown) was developed by Nektar Therapeutics.Exubera is not currently available. It was submitted to the FDA for approval in March of this year and could be approved before the end of the year. It's planned to be marketed for patients with either type 1 or type 2 diabetes.
For a summary of the study:
Study: Inhaled insulin works for type 1
For the study itself (abstract only, subscription required for full article):
Use of Inhaled Insulin in a Basal/Bolus Insulin Regimen in Type 1 Diabetic Subjects
Pfizer's news releases:
Data Presented at American Diabetes Association Scientific Sessions Support Exubera® Efficacy and Safety Profile in Type 1 and Type 2 Diabetes
also:
Pfizer and the Sanofi-Aventis Group Seek Approval to Market Exubera® in the United States