In a healthy person, the body senses insulin need and dispenses the hormone without the person's conscious thought or action. Future technologies may one day be able to mimic this unconscious sensing and dispensing of insulin for people with diabetes.
Last week, medical science moved a step closer to such a "closed loop" system with the FDA's approval of Medtronic's insulin pump and glucose monitoring system.
The MiniMed Paradigm® REAL-Time Insulin Pump and Continuous Glucose Monitoring System sends glucose readings every 5 minutes from a sensor to the pump. These continuous readings allow a user to monitor their glucose throughout the day. The system includes an alarm that vibrates or sounds when blood glucose is too high or too low, especially useful when sleeping. Action can be taken to improve glucose levels after a fingerstick confirmation.
 "Integrating an insulin pump with REAL-Time CGM is a major step toward the development of a “closed-loop” insulin delivery system that may one day mimic some functions of the human pancreas."
"Integrating an insulin pump with REAL-Time CGM is a major step toward the development of a “closed-loop” insulin delivery system that may one day mimic some functions of the human pancreas."- Medtronic's press release
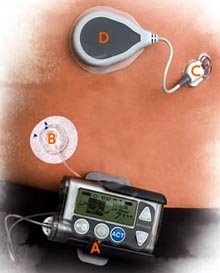
MiniMed's REAL-Time System is shown at right:
A - Insulin pump
B - Insulin delivery
C - Glucose sensor
D - Transmitter
If you're interested in just continuous glucose monitoring (CGM) without a pump, MiniMed currently sells the Guardian® RT CGM System.
Or you can buy the MiniMed Paradigm 522/722 insulin pump and use it without the glucose monitoring system. Later, if you decide to try continuous monitoring, you can purchase the REAL-Time Continuous Glucose Monitoring Kit, designed to be used with those pumps. The monitoring kit should be available this summer.
For Medtronic's press release:
Medtronic Receives FDA Approval for World's First Insulin Pump with Real-Time Continuous Glucose Monitoring